Ensuring quality and safe use, Govt notifies four common medical devices as drugs for regulation
New Delhi, December 10: Moving a step ahead, the health ministry has notified medical devices such as nebulizers, blood pressure monitors, digital thermometers and glucometers as drugs under the purview of the Drugs and Cosmetics Act. The decision was taken to ensure their quality and performance.
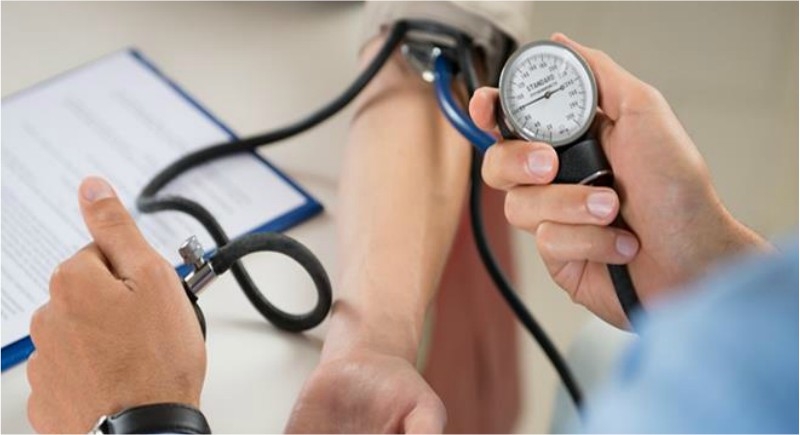
In a notification asserted by the Ministry of Health, it has been notified, “The Ministry of Health has through a notification dated December 3, specified devices intended for use in humans for internal or external use in the diagnosis, treatment, mitigation or prevention of disease or disorder in human beings or animals, to be included in the definition of drug under the Drugs and Cosmetics Act, 1940; effective from January 1, 2020.”
Once the proposal gets approved, it would mean companies which are engaged in manufacture and import of these equipment will have to seek necessary permission or license from the Drug Controller General of India January 1, 2020 onward.
All these devices will have to be registered under the quality parameters prescribed under Medical Devices Rules, 2017 and other standards set by the Bureau of Indian Standard (BIS) certification.
The Drug Technical Advisory Body (DTAB), India's highest drug advisory body, had approved the proposal to include nebulizers, blood pressure monitoring devices, digital thermometers and glucometers under the purview of the Drug law.
Currently, only 23 medical devices are monitored for quality by the country's drug regulator. With these four new devices being notified, 27 medical devices now fall under the definition of drugs under the Act. Other medical equipment are sold without any quality checks or clinical trials.
The health ministry has proposed expanding the list of devices in eight new categories, under the definition of 'drugs' to bring them under the purview of the Drugs and Cosmetics Act, 1940. The eight categories include implantable medical devices, MRI equipment, CT scan equipment, defibrillators, dialysis machines, PET equipment, X-ray machines and bone marrow cell separator.
The proposal to bring high-end medical devices like implants, X-ray machines, MRI and CT scan equipment, dialysis machines under the purview of the drug law is being deliberated.