IMA at it again! Objects Patanjali Ayurved's claim on Coronil
Total Views | 125
New Delhi, February 23: After raising questions on Ayush Ministry's Ayurvedic treatment for COVID-19, Indian Medical Association (IMA) has now taken the objection against Ayurvedic medicine Patanjali's Coronil. It has expressed shock over the "blatant lie of WHO certification" for the Coronil tablet.

What is the matter?
Ramdev baba, yoga guru and co-founder of Patanjali, on 19 February released a scientific research paper prepared by the Patanjali Research Institute on the "first evidence-based ayurvedic medicine" against coronavirus named Coronil. The paper was launched in the presence of Union ministers Harsh Vardhan and Nitin Gadkari at an event organised by Patanjali. During the lunch, Ramdev baba said, Coronil tablet had received certification from the Ayush Ministry as a medicine supporting COVID-19 treatment as per the World Health Organization's certification scheme.
However, WHO, clarifies that it has not reviewed or certified the effectiveness of any traditional medicine for the treatment of COVID-19. WHO's the regional office for South-East Asia posted on its official Twitter handle, "@WHO has not reviewed or certified the effectiveness of any traditional medicine for the treatment #Covidl9."

Following this, Patanjali's managing director Acharya Balkrishna later clarified the certification through a tweet saying, "We want to clarify to avoid confusion that our WHO GMP compliant COPP certificate to Coronil is issued by DCGI, Government of India. It is clear that WHO does not approve or disapprove of any drugs. WHO works for building a better, healthier future for people all over the world (sic)."

It should be noted, that the certificate of a pharmaceutical product (abbreviated: CPP) is a certificate issued in the format recommended by the World Health Organization (WHO), which establishes the status of the pharmaceutical product and the applicant for this certificate in the exporting country. It is issued for a single product because manufacturing arrangements and approved information for different pharmaceutical forms and strengths can vary.
What IMA Said
In a statement, IMA said, "Indian Medical Association is shocked to note the blatant lie of WHO certification for a secret medicine launched by Yoga guru Baba Ramdev's Patanjali Ayurved in the presence of Hon'ble Health Minister Harsh Vardhan." IMA has also demanded clarification from Health Minister Harsh Vardhan for promoting the Coronil tablet.
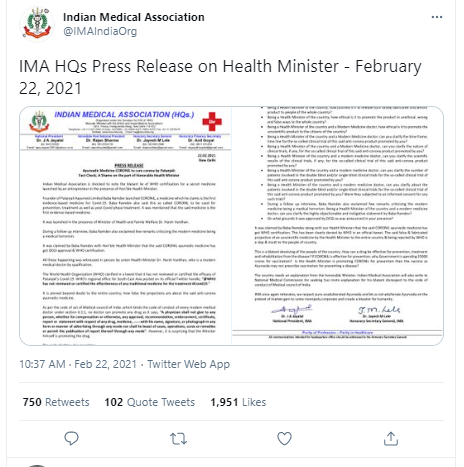
Just like earlier, the British-oriented medical association has asked 9 questions to the Health Minister Harsh Vardhan.
1. Being Health Minister of the country, how justified is it to release such falsely fabricated unscientific product to people of the whole country
2. How appropriate a rationale is it to release such false projection in front of the whole country?
3. How ethical was it to promote the product in unethical, wrong, and false ways?
4. How ethical is it to promote the unscientific product to citizens of the country?
5. Can you clarify the time frame timeline for the so-called clinical trial of this said anti-corona product promoted by you?
6. Can you clarify the nature of the clinical trials?
7. Can you clarify the scientific result of the clinical trials?
8. IMA has also sought clarifications on the timeline for the clinical trial of Coronil and whether patients were involved in the double-blind and/or single-blind clinical trials.
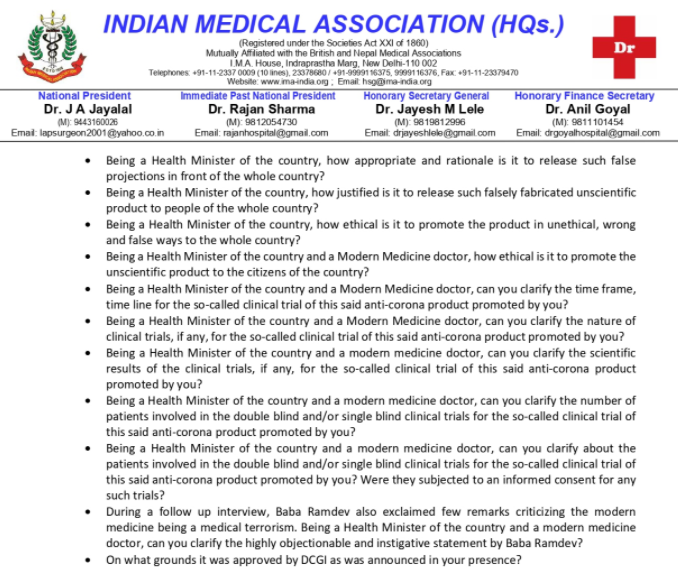
Pointing out at No doctor can promote any drug under Medical Council of India, section 6:1:1, IMA also has gave a warning to helth minister saying , "It also write to National Medical Commission for seeking Suo moto explanation for his blatant disrespect to the code of conduct of Medical council of India."
"A physician shall not give to any person, whether for compensation or otherwise, any approval, recommendation, endorsement, certificate, report or statement with respect of any drug, medicine, with his name, signature, or photograph in any form or manner of advertising through any mode nor shall he boast of cases, operations, cures or remedies or permit the publication of report thereof through any mode," the act reads.
In the end it said, "IMA once again reiterates, we respect pure unadulterated Ayurveda and let us not adulterate Ayurveda on the pretext of market gain to some monopoly corporate and create a disaster for humanity."
.
.